Fuel efficiency
Fuel efficiency is a form of thermal efficiency, meaning the efficiency of a process that converts chemical potential energy contained in a carrier fuel into kinetic energy or work. Overall fuel efficiency may vary per device, which in turn may vary per application, and this spectrum of variance is often illustrated as a continuous energy profile. Non-transportation applications, such as industry, benefit from increased fuel efficiency, especially fossil fuel power plants or industries dealing with combustion, such as ammonia production during the Haber process.
|
In the context of transport, fuel economy is the energy efficiency of a particular vehicle, and is given as a ratio of distance travelled per unit of fuel consumed. Fuel efficiency is expressed in miles per gallon (mpg) (prevalent in the USA and UK, using their respective definitions of a gallon) or kilometres per litre (km/L) (prevalent in the Netherlands and in several Latin American or Asian countries such as Brazil, India and Japan). The reciprocal ratio, "fuel consumption", is usually expressed in liters per 100 kilometers (L/100 km) (common in Europe, Canada, New Zealand and Australia) or litres per mil (Norway/Sweden).
Variations on a vehicle's fuel efficiency include weight-specific efficiency for freight, and passenger-specific efficiency (vehicle efficiency / number of passengers).
Vehicle design
Fuel efficiency is dependent on many parameters of a vehicle, including its engine parameters, aerodynamic drag, weight, and rolling resistance. There have been advances in all areas of vehicle design in recent decades.
Hybrid vehicles use two or more power sources for propulsion. In many designs, a small combustion engine is combined with electric motors. Kinetic energy which would otherwise be lost to heat during braking is recaptured as electrical power to improve fuel efficiency.
Fleet efficiency
Fleet efficiency describes the average efficiency of a population of vehicles. Technological advances in efficiency may be offset by a change in buying habits with a propensity to heavier vehicles, which are less efficient, all else being equal.
Energy efficiency terminology
Energy efficiency is similar to fuel efficiency but the input is usually in units of energy such as British thermal units (BTU), megajoules (MJ), gigajoules (GJ), kilocalories (kcal), or kilowatt-hours (kW·h). The inverse of "energy efficiency" is "energy intensity", or the amount of input energy required for a unit of output such as MJ/passenger-km (of passenger transport), BTU/ton-mile (of freight transport, for long/short/metric tons), GJ/t (for steel production), BTU/(kW·h) (for electricity generation), or litres/100 km (of vehicle travel). Litres per 100 km is also a measure of "energy intensity" where the input is measured by the amount of fuel and the output is measured by the distance travelled. For example: Fuel economy in automobiles.
Given a heat value of a fuel, it would be trivial to convert from fuel units (such as litres of gasoline) to energy units (such as MJ) and conversely. But there are two problems with comparisons made using energy units:
- There are two different heat values for any hydrogen-containing fuel which can differ by several percent (see below).
- When comparing transportation energy costs, it must be remembered that a kilowatt hour of electric energy may require an amount of fuel with heating value of 2 or 3 kilowatt hours to produce it.
Energy content of fuel
The specific energy content of a fuel is the heat energy obtained when a certain quantity is burned (such as a gallon, litre, kilogram). It is sometimes called the heat of combustion. There exists two different values of specific heat energy for the same batch of fuel. One is the high (or gross) heat of combustion and the other is the low (or net) heat of combustion. The high value is obtained when, after the combustion, the water in the exhaust is in liquid form. For the low value, the exhaust has all the water in vapor form (steam). Since water vapor gives up heat energy when it changes from vapor to liquid, the liquid water value is larger since it includes the latent heat of vaporization of water. The difference between the high and low values is significant, about 8 or 9%. This accounts for most of the apparent discrepancy in the heat value of gasoline. In the U.S. (and the table below) the high heat values have traditionally been used, but in many other countries, the low heat values are commonly used.
| Fuel type | MJ/L | MJ/kg | BTU/imp gal | BTU/US gal | Research octane number (RON) |
|---|---|---|---|---|---|
| Regular gasoline/petrol | 34.8 | ~47 | 150,100 | 125,000 | Min. 91 |
| Premium gasoline/petrol | ~46 | Min. 95 | |||
| Autogas (LPG) (60% propane and 40% butane) | 25.5–28.7 | ~51 | 108–110 | ||
| Ethanol | 23.5 | 31.1[1] | 101,600 | 84,600 | 129 |
| Methanol | 17.9 | 19.9 | 77,600 | 64,600 | 123 |
| Gasohol (10% ethanol and 90% gasoline) | 33.7 | ~45 | 145,200 | 121,000 | 93/94 |
| E85 (85% ethanol and 15% gasoline) | 33.1 | 44 | 108,878 | 90,660 | 100–105 |
| Diesel | 38.6 | ~48 | 166,600 | 138,700 | N/A (see cetane) |
| BioDiesel | 35.1 | 39.9 | 151,600 | 126,200 | N/A (see cetane) |
| Vegetable oil (using 9.00 kcal/g) | 34.3 | 37.7 | 147,894 | 123,143 | |
| Aviation gasoline | 33.5 | 46.8 | 144,400 | 120,200 | 80-145 |
| Jet fuel, naphtha | 35.5 | 46.6 | 153,100 | 127,500 | N/A to turbine engines |
| Jet fuel, kerosene | 37.6 | ~47 | 162,100 | 135,000 | N/A to turbine engines |
| Liquefied natural gas | 25.3 | ~55 | 109,000 | 90,800 | |
| Liquid hydrogen | 9.3 | ~130 | 40,467 | 33,696 |
Neither the gross heat of combustion nor the net heat of combustion gives the theoretical amount of mechanical energy (work) that can be obtained from the reaction. (This is given by the change in Gibbs free energy, and is around 45.7 MJ/kg for gasoline.) The actual amount of mechanical work obtained from fuel (the inverse of the specific fuel consumption) depends on the engine. A figure of 17.6 MJ/kg is possible with a gasoline engine, and 19.1 MJ/kg for a diesel engine. See Brake specific fuel consumption for more information.
Fuel efficiency of vehicles
The fuel efficiency of vehicles can be expressed in more ways:
- Fuel consumption is the amount of fuel used per unit distance; for example, litres per 100 kilometres (L/100 km). In this case, the lower the value, the more economic a vehicle is (the less fuel it needs to travel a certain distance); this is the measure generally used across Europe (except the UK and Denmark - see below), New Zealand, Australia and Canada. Also in Uruguay, Paraguay, Guatemala, Colombia, Japan, China, and Madagascar., as also in post-Soviet space.
- Fuel economy is the distance travelled per unit volume of fuel used; for example, kilometres per litre (km/L) or miles per gallon (MPG), where 1 MPG (imperial) = 0.354013 km/L. In this case, the higher the value, the more economic a vehicle is (the more distance it can travel with a certain volume of fuel). This measure is popular in the USA and the UK (mpg), but in Europe, India, Japan and Latin America the metric unit km/L is used instead.
Converting from mpg or to L/100 km (or vice versa) involves the use of the reciprocal function, which is not distributive. Therefore, the average of two fuel economy numbers gives different values if those units are used, because one of the functions is reciprocal, thus not linear. If two people calculate the fuel economy average of two groups of cars with different units, the group with better fuel economy may be one or the other. However, from the point of energy used as a shared method of measure, the result shall be the same in both the cases.
The formula for converting to miles per US gallon (3.785 L) from L/100 km is 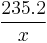 , where
, where 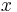 is value of L/100 km. For miles per Imperial gallon (4.546 L) the formula is
is value of L/100 km. For miles per Imperial gallon (4.546 L) the formula is 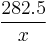 .
.
In Europe, the two standard measuring cycles for "litre/100 km" value are "urban" traffic with speeds up to 50 km/h from a cold start, and then "extra urban" travel at various speeds up to 120 km/h which follows the urban test. A combined figure is also quoted showing the total fuel consumed in divided by the total distance traveled in both tests. A reasonably modern European supermini and many mid-size cars, including station wagons, may manage motorway travel at 5 L/100 km (47 mpg US/56 mpg imp) or 6.5 L/100 km in city traffic (36 mpg US/43 mpg imp), with carbon dioxide emissions of around 140 g/km.
An average North American mid-size car travels 21 mpg (US) (11 L/100 km) city, 27 mpg (US) (9 L/100 km) highway; a full-size SUV usually travels 13 mpg (US) (18 L/100 km) city and 16 mpg (US) (15 L/100 km) highway. Pickup trucks vary considerably; whereas a 4 cylinder-engined light pickup can achieve 28 mpg (8 L/100 km), a V8 full-size pickup with extended cabin only travels 13 mpg (US) (18 L/100 km) city and 15 mpg (US) (15 L/100 km) highway.
European-built cars are generally more fuel-efficient than American vehicles. While Europe has many higher efficiency diesel cars, European gasoline vehicles are on average also more efficient than gasoline-powered vehicles in the USA. Most European vehicles cited in the CSI study run on diesel engines, which tend to achieve greater fuel efficiency than gas engines. Selling those cars in the United States is difficult because of emission standards, notes Walter McManus, a fuel economy expert at the University of Michigan Transportation Research Institute. “For the most part, European diesels don’t meet U.S. emission standards,” McManus said in 2007. Another reason why many European models are not marketed in the United States is that labor unions object to having the big 3 import any new foreign built models regardless of fuel economy while laying off workers at home.[2]
An interesting example of European cars' capabilities of fuel economy is the microcar Smart Fortwo cdi, which can achieve up to 3.4 l/100 km (69.2 mpg US) using a turbocharged three-cylinder 41 bhp (30 kW) Diesel engine. The Fortwo is produced by Daimler AG and is currently only sold by one company in the United States. Furthermore, the current (and to date already 10 year old) world record in fuel economy of production cars is held by the Volkswagen Group, with special production models (labeled "3L") of the Volkswagen Lupo and the Audi A2, consuming (NEDC ratified) as little as 2.99 litres of diesel fuel per 100 kilometres (78 miles per US gallon or 94 miles per Imperial gallon).
Diesel engines generally achieve greater fuel efficiency than petrol (gasoline) engines. Passenger car diesel engines have energy efficiency of up to 41% but more typically 30%, and petrol engines of up to 37.3%, but more typically 20%. That is one of the reasons why diesels have better fuel efficiency than equivalent petrol cars. A common margin is 25% more miles per gallon for an efficient turbodiesel. For example, the current model Skoda Octavia, using Volkswagen engines, has a combined European fuel efficiency of 41.3 mpg for the 105 bhp (78 kW) petrol engine and 52.3 mpg for the 105 bhp (78 kW) — and heavier — diesel engine. The higher compression ratio is helpful in raising the energy efficiency, but diesel fuel also contains approximately 10% more energy per unit volume than gasoline which contributes to the reduced fuel consumption for a given power output.
Fuel efficiency in microgravity
How fuel combusts affects how much energy is produced. The National Aeronautics and Space Administration (NASA) has investigated fuel consumption in microgravity.
The common distribution of a flame under normal gravity conditions depends on convection, because soot tends to rise to the top of a flame, such as in a candle, making the flame yellow. In microgravity or zero gravity, such as an environment in outer space, convection no longer occurs, and the flame becomes spherical, with a tendency to become more blue and more efficient. There are several possible explanations for this difference, of which the most likely one given is the hypothesis that the temperature is evenly distributed enough that soot is not formed and complete combustion occurs.[3] Experiments by NASA in microgravity reveal that diffusion flames in microgravity allow more soot to be completely oxidised after they are produced than diffusion flames on Earth, because of a series of mechanisms that behaved differently in microgravity when compared to normal gravity conditions.[4] Premixed flames in microgravity burn at a much slower rate and more efficiently than even a candle on Earth, and last much longer.[5]
Transportation
Fuel efficiency in transportation
Vehicle efficiency and transportation pollution
Fuel efficiency directly affects emissions causing pollution by affecting the amount of fuel used. However, it also depends on the fuel source used to drive the vehicle concerned. Cars can, for example, run on a number of fuel types other than gasoline, such as natural gas, LPG or biofuel or electricity which creates various quantities of atmospheric pollution.
A kilogram of carbon, whether contained in petrol, diesel, kerosene, or any other hydrocarbon fuel in a vehicle, leads to approximately 3.6Kg of CO2 emissions.[6] Due to the carbon content of gasoline, its combustion emits 2.3 Kg/l (19.4 lb/US gal) of CO2; since diesel fuel is more energy dense per unit volume, diesel emits 2.6 Kg/l (22.2 lb/US gal).[6] This figure is only the CO2 emissions of the final fuel product and does not include additional CO2 emissions created during the drilling, pumping, transportation and refining steps required to produce the fuel. Additional measures to reduce overall emission includes improvements to the efficiency of air conditioners, lights and tires.
Driving technique
Many tips are available from various government and local sources to help drivers improve their fuel efficiency.
There is a growing community of enthusiasts known as hypermilers who develop and practice driving techniques to increase fuel efficiency and reduce consumption. Hypermilers have broken records of fuel efficiency, for example, achieving 109 miles per gallon in a Prius. In non-hybrid vehicles these techniques are also beneficial. Hypermiler Wayne Gerdes can get 59 MPG in a Honda Accord and 30 MPG in an Acura MDX.[7]
The most efficient machines for converting energy to rotary motion are electric motors, as used in electric vehicles. However, electricity is not a primary energy source so the efficiency of the electricity production has also to be taken into account. Currently railway trains can be powered using electricity, delivered through an additional running rail, overhead catenary system or by on-board generators used in diesel-electric locomotives as common on the UK rail network. Pollution produced from centralised generation of electricity is emitted at a distant power station, rather than "on site". Some railways, such as the French SNCF and Swiss federal railways derive most, if not 100% of their power, from hydroelectric or nuclear power stations, therefore atmospheric pollution from their rail networks is very low. This was reflected in a study by AEA Technology between a Eurostar train and airline journeys between London and Paris, which showed the trains on average emitting 10 times less CO2, per passenger, than planes, helped in part by French nuclear generation.[8] This can be changed using more renewable sources for electric generation.
In the future hydrogen cars may be commercially available. Powered either through chemical reactions in a fuel cell that create electricity to drive very efficient electrical motors or by directly burning hydrogen in a combustion engine (near identically to a natural gas vehicle, and similarly compatible with both natural gas and gasoline); these vehicles promise to have near zero pollution from the tailpipe (exhaust pipe). Potentially the atmospheric pollution could be minimal, provided the hydrogen is made by electrolysis using electricity from non-polluting sources such as solar, wind or hydroelectricity or thermochemically by the use of the Thorium fuel cycle in a molten salt reactor.
In any process, it is vitally important to account for all of the energy used throughout the process. Thus, in addition to the energy cost of the electricity or hydrogen production, we must also account for transmission and/or storage losses to support large-scale use of such vehicles. For this reason the use of the idea "zero pollution" should be avoided.
See also
References
- ^ Calculated from heats of formation. Does not correspond exactly to the figure for MJ/L divided by density.
- ^ EuropeVsUS Efficiency
- ^ CFM-1 experiment results, National Aeronautics and Space Administration, April 2005.
- ^ LSP-1 experiment results, National Aeronautics and Space Administration, April 2005.
- ^ SOFBAL-2 experiment results, National Aeronautics and Space Administration, April 2005.
- ^ a b "Emission Facts: Average Carbon Dioxide Emissions Resulting from Gasoline and Diesel Fuel". Office of Transportation and Air Quality. United States Environmental Protection Agency. February 2005. http://www.epa.gov/OMS/climate/420f05001.htm. Retrieved 2009-07-28.
- ^ Gaffney, Dennis (2007-01-01). "This Guy Can Get 59 MPG in a Plain Old Accord. Beat That, Punk.". Mother Jones. http://www.motherjones.com/news/feature/2007/01/king_of_the_hypermilers.html. Retrieved 2007-04-20.
- ^ European Federation for Transport and Environment
External links
- US Government website on fuel economy
- UK DfT comparisons on road and rail
- NASA Offers a $1.5 Million Prize for a Fast and Fuel-Efficient Aircraft
- Spritmonitor.de "the most fuel efficient cars" - Database of thousands of (mostly German) car owners' actual fuel consumption figures
- Independent testing proves fuel savings of innovative engine